NAL External Audit at Hazera
The annual NAL external audit is one of the most important milestones in our QA calendar. It’s an intense, detail-oriented week that requires accuracy, teamwork, and readiness across every department.
NAL stands for Naktuinbouw Authorized Laboratory. Its accreditation ensures that seed testing laboratories operate with high reliability and quality standards.
The goal is to guarantee that seeds meet strict quality requirements before commercialization, providing confidence to customers and regulatory bodies. This accreditation reflects compliance with Naktuinbouw conditions and international protocols for seed quality testing.
I approach this week together with Michal Efrat – Quality Assurance Manager IL, whose experience and guidance are essential in ensuring everything runs smoothly. This is how our audit week unfolded.
Sunday – First preparations & system alignment
As the workweek begins on Sunday, the NAL audit preparations are already underway. Together with Michal, I review the full audit plan and prioritize what needs attention before the auditors connect.
Our focus areas include:
- Ensuring all documentation is updated and easily accessible
- Reviewing corrective actions from previous audits – both internal and external
- Validating training files and operator competencies
- Confirming traceability across the labs and the processing plant
Having conducted remote audits consistently over the past three years, we are well-versed in NAL’s expectations and highly experienced with both the process flows and the technical challenges associated with remote audits. All our remote audit equipment – a clearly time-lined agenda in Teams, phone camera, Jabra speaker, and backup devices – is prepared in advance so we can concentrate fully on content rather than logistics.
Sunday ends with a solid overview and clear direction for the next day.
Monday – Documentation completion
Monday is dedicated to advancing one of our most important long-term improvements: the transition to our new documentation system, WIN. We focus on organizing existing materials, mapping, and ensuring the new structure fully supports daily operations.
The WIN system brings significant advantages to QA and all operational units:
- Centralized and controlled — documents are stored in one place, easy to find, and always up to date.
- Automated workflows — approvals and updates run smoothly, supporting compliance with ISO, NAL, and corporate standards.
- Multi-language support — improving accessibility for all teams.
- Smart navigation — links between related documents and role-based access ensure users quickly find only what’s relevant to their function.
This marks a meaningful shift toward more efficient, transparent, and aligned documentation management.
In addition, we schedule a focused session with key departments to address Non-Conformities (NC), support their analysis, and ensure follow-up on corrective actions that drive real change. This collaboration strengthens ownership and improves solution effectiveness.
By the end of Monday, we’ve made strong progress: the documentation system is well-structured, and teams are aligned on actions, setting a solid foundation for the upcoming audit and future improvements.
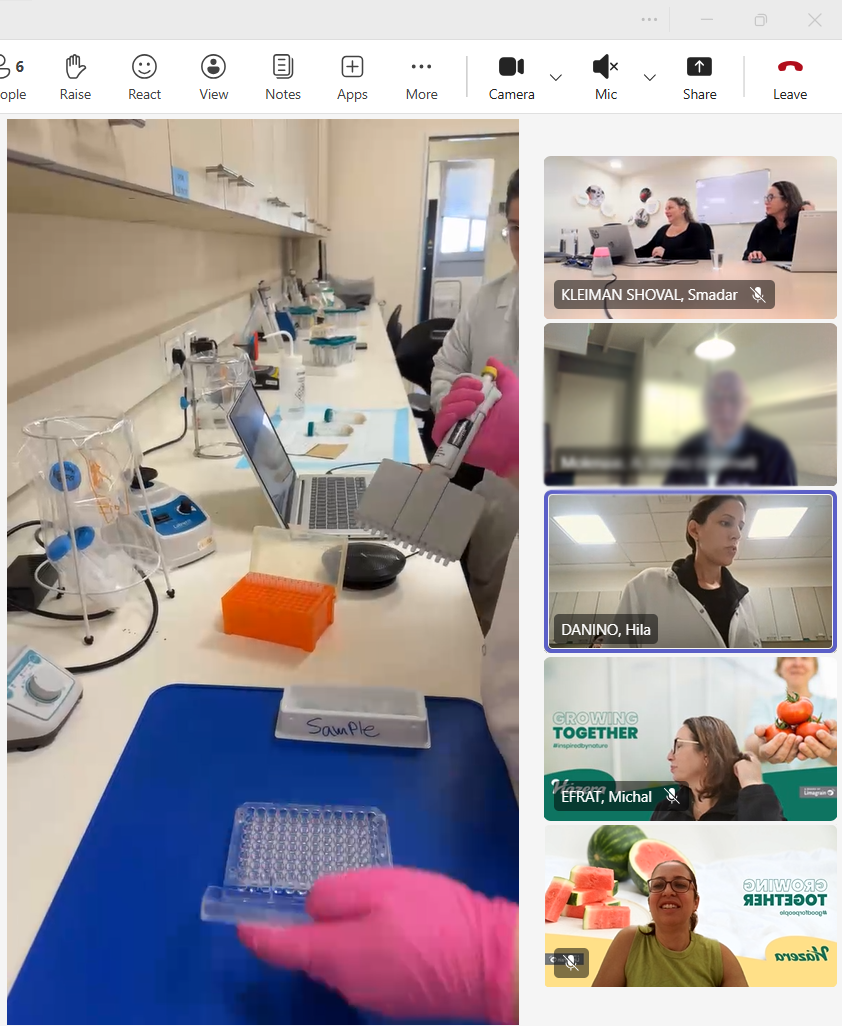
Tuesday – Audit day 1: Health Lab
The first audit day starts with an unexpected twist: one of the professional auditors is sick and unable to join. Fortunately, a replacement is arranged quickly, and we begin the session without delays.
Using all the tools, the auditors are guided through the Health Lab.
Focus points include:
- Sample reception & labeling
- Equipment qualification
- Method demonstration
- Training files for each Health Lab technician
- Documentation and data integrity
We also make sure to show as many Health Lab methods as possible within the time frame, giving the auditors a complete and realistic view of our routine QC work.
The day ends with positive feedback – a good start.
Wednesday – Audit day 2: Germination Lab
The second day focuses on the Germination Lab.
We demonstrate:
- Accredited germination methods
- Environmental monitoring
- Scoring and record keeping
- Sample identification
- Training and qualification of germination technicians
We walk the auditors through multiple workflows, ensuring they get a full picture of our capabilities.
Thursday – Audit day 3: Processing Plant
The final day of the audit focuses on the Processing Plant, with a more targeted and concise scope.
The auditors review two core methods:
- TSW (1000 seed weight)
- The sampling process
We demonstrate both methods clearly through the remote setup. The auditors also place strong emphasis on two essential QA elements:
- Training and competency of plant employees, including qualification for each task
- Equipment calibration and maintenance, ensuring accuracy and reliability over time
The team provides all required documents and explanations, and the session flows smoothly from start to finish. The auditors show strong engagement throughout, and the review concludes on a positive note, reinforcing the solid work and professionalism of the plant team.
Looking back
This NAL audit week was intense but deeply rewarding.
Working closely with all the operational teams from QC and the processing plant showed once again how much preparation, communication, and teamwork matter.
The effort was truly cross-functional, connecting QA with every part of the business to ensure compliance, efficiency, and customer trust.
Despite the limitations of remote auditing, we provided a complete, transparent, and confident demonstration of our work. We can proudly say:
It was a successful audit and a valuable learning experience – The observations shared by the auditors will help us further improve our processes to ensure the highest quality of operations and products before delivering seeds to our customers.